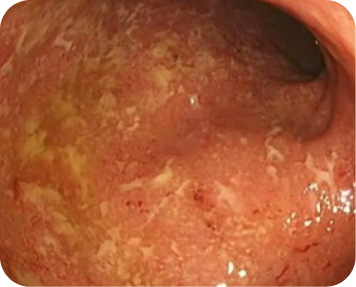
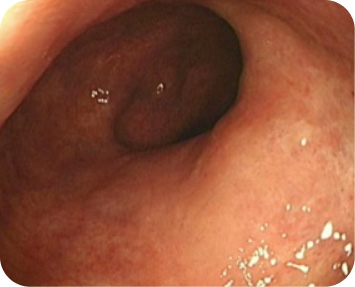
For adults with moderately to severely active ulcerative colitis (UC).
WHY CHOOSE
TREMFYA®?
TREMFYA®
LEGACY
14+
YEARS
of combined clinical research*
6+
YEARS
on the market across multiple indications
*Based on a clinical trial of TREMFYA® initiated in 2009.
TREMFYA® Safety Information
TREMFYA® may cause serious side effects, including:
- Serious allergic reactions. Stop using TREMFYA® and get emergency medical help right away if you develop any of the following symptoms of a serious allergic reaction:
- fainting, dizziness, feeling lightheaded (low blood pressure)
- swelling of your face, eyelids, lips, mouth, tongue or throat
- trouble breathing or throat tightness
- chest tightness
- skin rash, hives
- itching
- Infections. TREMFYA® is a medicine that may lower the ability of your immune system to fight infections and may increase your risk of infections. Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with TREMFYA® and may treat you for TB before you begin treatment with TREMFYA® if you have a history of TB or have active TB. Your healthcare provider should watch you closely for signs and symptoms of TB during and after treatment with TREMFYA®. Tell your healthcare provider right away if you have an infection or have symptoms of an infection, including:
- fever, sweats, or chills
- cough
- shortness of breath
- blood in your phlegm (mucus)
- muscle aches
- warm, red, or painful skin or sores on your body different from your psoriasis
- weight loss
- diarrhea or stomach pain
- burning when you urinate or urinating more often than normal
These are not all the possible side effects of TREMFYA®.
Please read the Important Safety Information located at the bottom of the screen and the Medication Guide for TREMFYA® to learn more about these and other risks for TREMFYA®. Discuss any questions you have with your doctor.
TREMFYA® may cause serious side effects, including:
- See “What is the most important information I should know about TREMFYA®?”
The most common side effects of TREMFYA® include:
- respiratory tract infections
- joint pain (arthralgia)
- fungal skin infections
- headache
- diarrhea
- herpes simplex infections
- injection site reactions
- stomach flu (gastroenteritis)
- bronchitis
These are not all the possible side effects of TREMFYA®. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
TREMFYA® may cause serious side effects, including:
- Serious allergic reactions. Stop using TREMFYA® and get emergency medical help right away if you develop any of the following symptoms of a serious allergic reaction:
- fainting, dizziness, feeling lightheaded (low blood pressure)
- swelling of your face, eyelids, lips, mouth, tongue or throat
- trouble breathing or throat tightness
- chest tightness
- skin rash, hives
- itching
- Infections. TREMFYA® is a medicine that may lower the ability of your immune system to fight infections and may increase your risk of infections. Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with TREMFYA® and may treat you for TB before you begin treatment with TREMFYA® if you have a history of TB or have active TB. Your healthcare provider should watch you closely for signs and symptoms of TB during and after treatment with TREMFYA®. Tell your healthcare provider right away if you have an infection or have symptoms of an infection, including:
- fever, sweats, or chills
- cough
- shortness of breath
- blood in your phlegm (mucus)
- muscle aches
- warm, red, or painful skin or sores on your body different from your psoriasis
- weight loss
- diarrhea or stomach pain
- burning when you urinate or urinating more often than normal
These are not all the possible side effects of TREMFYA®.
Please read the Important Safety Information located at the bottom of the screen and the Medication Guide for TREMFYA® to learn more about these and other risks for TREMFYA®. Discuss any questions you have with your doctor.
TREMFYA® may cause serious side effects, including:
- See “What is the most important information I should know about TREMFYA®?”
The most common side effects of TREMFYA® include:
- respiratory tract infections
- joint pain (arthralgia)
- fungal skin infections
- headache
- diarrhea
- herpes simplex infections
- injection site reactions
- stomach flu (gastroenteritis)
- bronchitis
These are not all the possible side effects of TREMFYA®. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.